Blood-thirsty bug bound by unravelling genes
 Close to 700 million people are infected with a single blood-sucking worm, but now researchers have probed its DNA for ways to fight back.
Close to 700 million people are infected with a single blood-sucking worm, but now researchers have probed its DNA for ways to fight back.
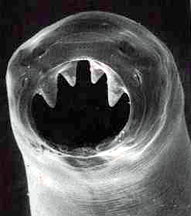
The hookworm, Necator americanus, is a parasitic invader which enters the body through bare feet.
Hookworms begin life in the soil before a barefoot host gives them a lift. Once inside the body the worms feed on blood, are able to avoid detection, cause anemia and in children; stunted growth and learning problems.
Researchers have now gained a point for the humans’ team, decoding the hookworm’s DNA to find out how it infects and survives in the body to aid in development of new therapies.
“We now have a more complete picture of just how this worm invades the body, begins feeding on the blood and successfully evades the host immune defences,” said senior author Makedonka Mitreva, PhD, assistant professor of medicine and of genetics at Washington University School of Medicine in St Louis.
“This information will accelerate development of new diagnostic tools and vaccines against the infection.”
There is nothing nice about the lifecycle of the hookworm.
The worm's eggs are excreted in the faeces of infected individuals, contaminating the soil. After the eggs hatch, immature worms called larvae enter the body through the feet. The worms travel through the bloodstream to the lungs, where they are coughed up and then swallowed, making their way to the small intestine. It is there that the worms mature and begin feeding on blood.
Repeated and excessive use of the common de-worming drug albendazole has led to mass treatment failures and drug resistance in some regions, researchers say.
The latest investigations have taken a molecular-level look at the way hookworms invade the body and evade the host's immune system. Researchers focused on possible genetic targets for vaccines or treatment.
While have a clearly negative effect in a majority of cases, hookworms have garnered recent attention for their possible use in the treatment of autoimmune diseases. This interest has been sparked by the discovery of the worms’ ability to trick the host’s immune response by suppressing molecules that promote inflammation.
“It is our hope that the new research can be used as a springboard not just to control hookworm infections but to identify anti-inflammatory molecules that have the potential to advance new therapies for autoimmune and allergic diseases,” Dr Mitreva said.









 Print
Print