TGA bans mesh implants
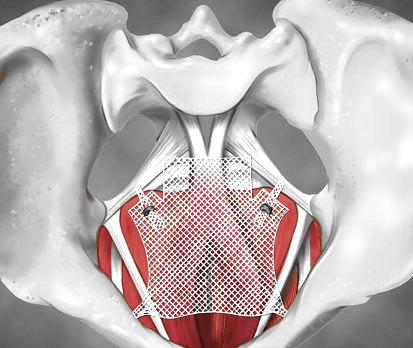 Transvaginal mesh products used to treat pelvic organ prolapse have been banned.
Transvaginal mesh products used to treat pelvic organ prolapse have been banned.
Australia’s medical devices regulator, the TGA, has ruled that the products are too risky, and removed them from the Australian Register of Therapeutic Goods (ARTG).
Some of the products are the subject of a large-scale lawsuit, but opinion on their usefulness has been divided.
The ban follows a review by the TGA of studies and clinical evidence, and the publication of the TGA’s Results of review into urogynaecological surgical mesh implants.
The regulator says the benefits of using transvaginal mesh products in the treatment of pelvic organ prolapse do not outweigh the risks these products pose to patients.
The TGA says there is a lack of adequate scientific evidence for it to be satisfied that the risks to patients associated with the use of mesh products as single incision mini-slings for the treatment of stress urinary incontinence are outweighed by their benefits.
It noted that ‘mini-slings’ are different devices to mid-urethral slings, which are not being removed from the ARTG.
The ban will take effect 20 working days after the notices are issued, 4 January 2018.
The products will remain on the ARTG until that date and may continue to be lawfully supplied until then.
There is also a 90-day window within which a review of the TGA's decisions in relation to the mesh products can be requested.
Since 2013, 45 devices have been removed from urogynaecological use by the TGA – 43 cancelled from the ARTG and a further two have been limited to non-urogynaecological procedures.








 Print
Print